The cell therapy isolator integrates cell culture-related functional components such as centrifuges and CO2 incubators to complete cell culture-related operations in a closed aseptic environment to meet the GMP production requirements of cell products.
Vertical laminar flow;
Built-in Vaporized Hydrogen Peroxide (VHPS) Generator for thorough decontamination;
Adopts dry VHPS technology to avoid condensation;
Unique concentration dynamiclly control technology to avoid the VHPS concentration is too high or too low;
Pressure control technology to minimize the risk of VHPS penerating in the package;
Temperature control during the decontamination process to minimize the high temperature affecting the cell viability;
The chamber cleanliness is monitored by the air sampler and particle counter;
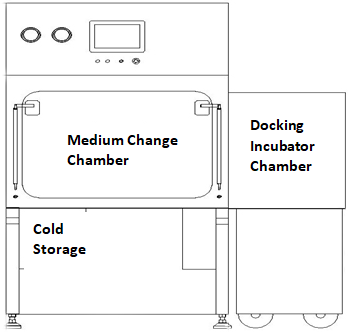
Movable cell incubator can avoid the cross contamination;
Automatic medium replacement modular can improve the efficiency and low the labor cost;
Modular design which is easily installed in class D background ;
Validation service: DQ,IQ,OQ,PQ,VHPS penetration test.
Cleanliness Class: ISO5 ClassA
HEPA Filters:H14, HEPA≥99.995%,@0.3um
Bio-decontamination time: approx. 2.5 hours,reach 6-log SAL
illumination: >500lux
Power Supply: 380V 50Hz
Pressure: 20~80Pa adjustable
Overall Dimensions (LxWxH):5500mm×1205mm×2600mm(Could be customoized)
Structure: SUS 316L and 15mm tempered glass
Incubator temperature: 37℃±0.2
Temperature resolution: 0.1℃
Temperature fluctuation: ±0.2℃
CO2 concentration: 5%
Cold storage temperature: 2-8℃
Rewarming temperature: 37℃
Medium replacement speed: 5min/dish
Noise≤65dB(A)
Designed for the users who require sterile conditions for R&D or pilot production of cell & gene products.
Technical specifications are subject to change without notice. Copyright reserved by Tailin.


 CN
CN












